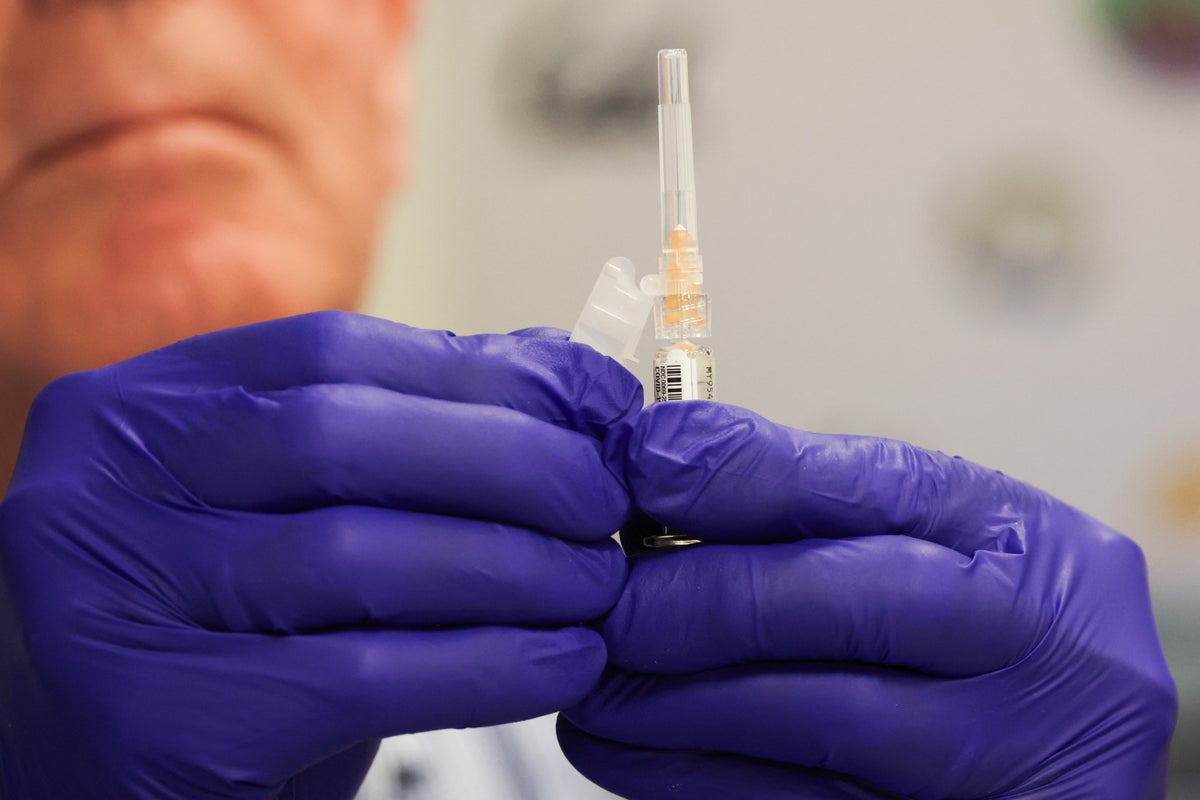
"The U.S. Food and Drug Administration is reportedly weighing whether it will place a black box warning label on COVID vaccines, according to CNN, despite research and real-world data demonstrating their safety. Black box warnings are the FDA's highest health warning for drugs and medical devices. Adding them to COVID vaccines would be an unprecedented action by the FDA and would likely result in significant reduction in use of the product, says physician Robert Hopkins, medical director of the National Foundation for Infectious Diseases."
"The report comes a week after a leaked memo, allegedly written by the FDA's chief medical and scientific officer Vinay Prasad, detailed plans to change COVID vaccine approvals and linked the deaths of 10 children to the vaccines, without evidence. In response to Scientific American, Department of Health and Human Services spokesperson Andrew Nixon dismissed the claims as pure speculation and said that the FDA takes very seriously any death that is attributed to a regulated medical product."
"Black box warnings, also called boxed warnings, are designed to inform people of major health risks associated with a drug, treatment or other medical intervention, including a vaccine, and generally indicate a risk of serious injury or life-threatening harm. Opioid painkillers carry such a warning, for example. The FDA has the power to add these labels, update them or remove them at any point as data becomes available to the agency."
FDA is reportedly considering placing a black box warning on COVID vaccines. Black box warnings are the agency's highest health warning and signal risk of serious injury or life-threatening harm. Adding such labels to COVID vaccines would be unprecedented and would likely reduce use substantially. It remains unclear whether any warning would apply to all authorized vaccines or to specific groups such as pregnant people or children. A leaked memo allegedly linked ten child deaths to vaccines, though no evidence supported that claim and HHS described the linkage as speculation. The FDA can add, update, or remove boxed warnings as new data emerge.
Read at www.scientificamerican.com
Unable to calculate read time
Collection
[
|
...
]