"FDA had promised to do a top-to-bottom safety review of the chemical abortion drug, but instead they've just greenlighted new versions of it for distribution,"
"I have lost confidence in the leadership at FDA."
"The FDA has very limited discretion in deciding whether to approve a generic drug,"
"By law, the Secretary of Health and Human Services must approve an application if it demonstrates that the generic drug is identical to the brand-name drug,"
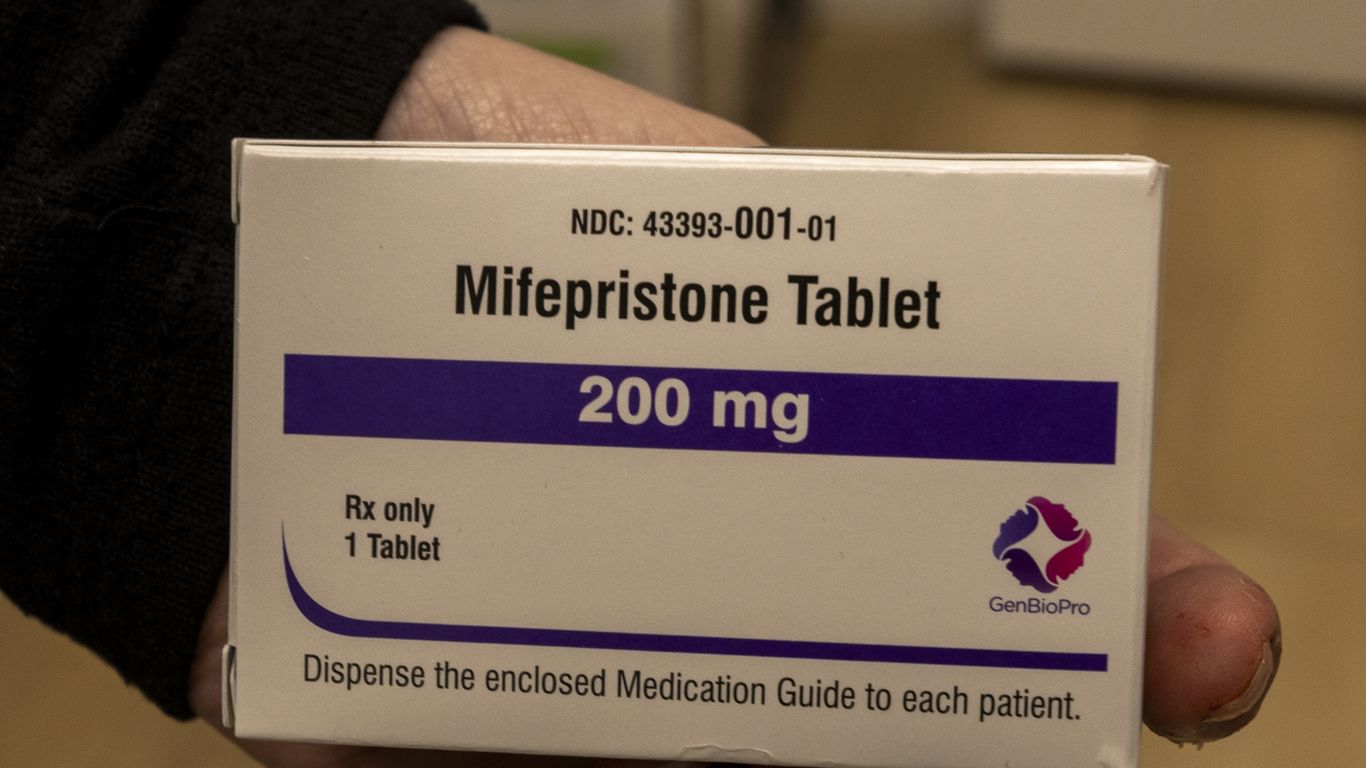
FDA approved a generic version of mifepristone manufactured by Evita Solutions, and Evita promoted the product on its website. Generic approval typically requires proving the copycat matches the original and gives the FDA limited discretion. Mifepristone’s use in terminating early pregnancy makes new approvals politically charged. Abortion opponents pointed to recent comments by Health Secretary Robert F. Kennedy Jr. and Makary about studying new safety limits and criticized the approval. Senator Josh Hawley and Marjorie Dannenfelser publicly condemned the decision. HHS responded that law constrains the FDA to approve identical generics and that the agency is studying safety risks.
Read at Axios
Unable to calculate read time
Collection
[
|
...
]