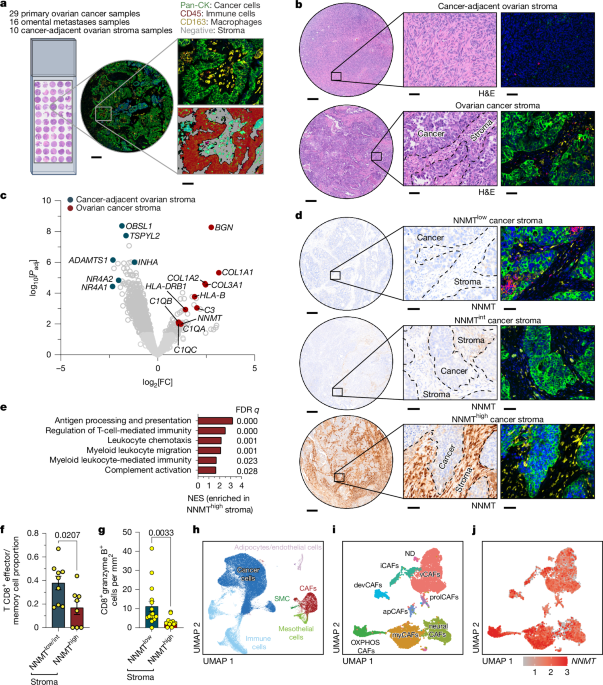
"Nicotinamide N-methyltransferase (NNMT) plays a crucial role in high-grade serous ovarian cancer by inducing H3K27me3 hypomethylation, which drives complement secretion from cancer-associated fibroblasts (CAFs). This process attracts immunosuppressive myeloid-derived suppressor cells (MDSCs) to the tumor. The absence of Nnmt in immunocompetent mice hampers tumor growth in various syngeneic models, showcasing an enhancement in CD8+ T cell activation. A high-throughput screening yield led to the development of a potent NNMT inhibitor that effectively reduces tumor burden and metastasis across different mouse cancer models, also improving immune checkpoint blockade efficacy."
"Targeting cancer-associated fibroblasts (CAFs) remains a challenge due to the lack of effective therapies. The investigation into NNMT reflects a novel approach in understanding CAFs' supportive roles in cancer progression."
"In multiple mouse cancer models, a specific NNMT inhibitor not only decreased tumor burden and metastasis but also significantly increased the effectiveness of immune checkpoint blockade therapies, highlighting the therapeutic potential of NNMT targeting."
"The mechanistic insights gained from this study suggest that by inhibiting NNMT, it is possible to disrupt the immune-suppressive tumor microenvironment by diminishing the recruitment of immunosuppressive myeloid-derived suppressor cells (MDSCs)."
Nicotinamide N-methyltransferase (NNMT) is implicated in the dynamics of high-grade serous ovarian cancer, where it induces H3K27me3 hypomethylation. This mechanism facilitates the secretion of complements from cancer-associated fibroblasts (CAFs), which in turn attracts immunosuppressive myeloid-derived suppressor cells (MDSCs) to the tumor environment. Observations in Nnmt knockout mouse models reveal impaired tumor growth and enhanced CD8+ T cell activation. Advances in high-throughput screening have led to a potent NNMT inhibitor, demonstrating a reduction in tumor burden and metastasis while enhancing immune checkpoint blockade efficacy in various cancer models.
Read at www.nature.com
Unable to calculate read time
Collection
[
|
...
]