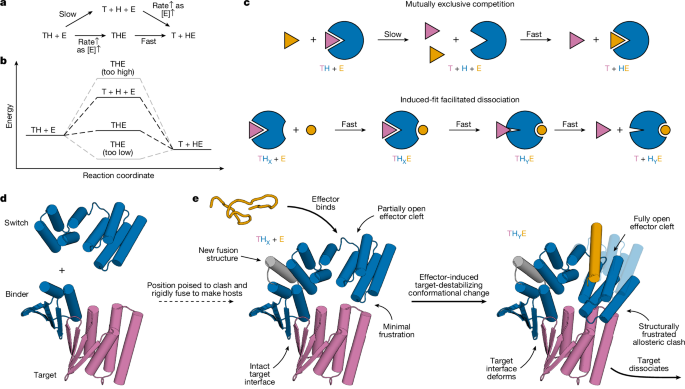
"Protein-protein interactions orchestrate much of biological function. High-affinity interactions enable protein circuits to respond to low concentrations of stimuli and to act potently on targets; fast exchange enables them to respond quickly to changes in stimuli. These two properties cannot usually be achieved simultaneously in binary interactions because they depend on the interaction off-rate in opposite ways: high affinity usually requires slow dissociation (low off-rate), whereas rapid exchange requires fast dissociation (high off-rate) (Supplementary Fig. 1)."
"Several natural systems exhibit 'facilitated dissociation'10,11,12,13,14,15,16,17,18,19,20, in which an effector (E) can bind to a target-host (TH) complex to form an excited ternary complex (THE)20,21,22,23,24,25,26 from which the target dissociates quickly (Fig. 1a-c). In such a system, the target can bind tightly to the host, yet can also be rapidly released by adding the effector27. In engineered DNA systems, the kinetic control afforded by an analogous phenomenon (toehold-mediated strand displacement) has enabled the construction of many complex functions28,29,"
High-affinity binding and rapid exchange are opposing requirements because they depend inversely on dissociation rates. Facilitated dissociation allows an effector to bind a target-host complex and form an excited ternary complex from which the target dissociates quickly, enabling tight binding that is also rapidly reversible upon effector addition. Analogous kinetic control in DNA via toehold-mediated strand displacement has enabled complex functions, but DNA systems poorly interface with biology. Protein binding and unbinding can couple directly to biological processes, yet a general approach to design kinetic control over protein interactions has been lacking.
Read at Nature
Unable to calculate read time
Collection
[
|
...
]